Logistics Supports a Healthy Pharma Industry

From managing distribution channels to boosting visibility and security, the pharmaceutical and life sciences supply chain mixes complexity with risk. Here are the technologies and strategies that inject efficiency into supply chains.
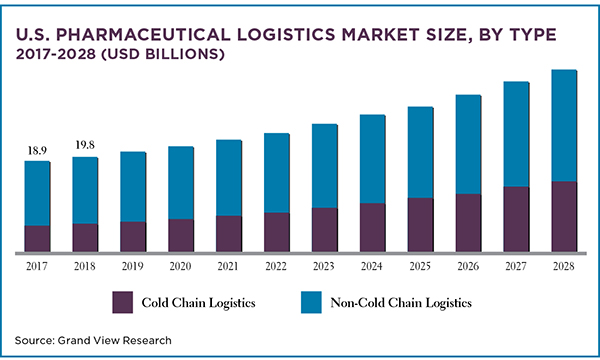
The market for pharmaceuticals and biopharmaceuticals is robust and growing and that’s driving demand for logistics support for these products. (Biopharmaceuticals are medicines and drugs produced using biotechnology or living things, especially cells and bacteria, in industrial processes.)
The global pharmaceutical manufacturing market, valued at US$324.42billion in 2019, is expected to grow at a compound annual growth rate of13.74% between 2020 to 2027, according to Grand View Research. Following along, the global pharmaceutical logistics market will grow by 8.5% annually between 2021 and 2028.
This growth is a positive for the individuals who rely on these medicines, as well as the companies that supply and distribute them. Yet it also poses challenges, including accurately forecasting demand, while containing costs and managing complicated transportation and cold-chain requirements even as capacity tightens. Security remains another concern, given that a truckload of medicine can be worth millions.
When working with over-the-counter medicines and healthcare products, a few other supply chain obstacles arise. One is managing the range of distribution channels, says Jeffrey Tucker, chief executive officer with Tucker Company Worldwide, a leader in life sciences transportation. Then, the penalties some larger retailers levy on shipments that miss on-time, in-full (OTIF) requirements can quickly cut into profits.
"The careful and planned execution of transportation is critical," Tucker adds.
A Dose of Technology
Technology plays an increasingly important role in the transportation and distribution of pharma products. Solutions that incorporate artificial intelligence (AI), robotics, automation, and the Internet of Things (IoT) offer advantages over many legacy systems, like conveyors and automated storage and retrieval systems, which often required a tremendous upfront investment of money, management time and attention, and warehouse space, says Jett Chitanand, director, global sales, healthcare, with Locus Robotics, a provider of innovative autonomous mobile robots. Moreover, the solutions often were designed to meet a specific need or demand.
"However, businesses and priorities change, making these investments less attractive," Chitanand says.
Many legacy supply chain monitoring systems also have tended to assemble information in siloes, leading to a loss of visibility. More than half (57%) of respondents to a recent survey by WBR Research and Controlant, which offers cold chain as a service, say they experienced a loss of visibility and bottlenecks in their supply chains of raw material and active pharmaceutical ingredients.
Despite the shortcomings of many previous solutions, earlier efforts to transform pharmaceutical supply chains tended to be "slow-moving," says Gisli Herjolfsson, co-founder and chief executive officer with Controlant.
That’s changing. The pandemic "starkly exposed major vulnerabilities in the pharmaceutical and medical products supply chains," says Per Hong, senior partner in the strategic operations practice at Kearney.
The pandemic will almost certainly reshape global life science supply chains to strengthen resiliency, ensure access to more local sources of capacity and supply, and manage allocations of vital products, he adds.
Indeed, one bright spot to the pandemic has been the urgency that spurred industry players to take an "all-hands-on-deck collaboration" approach to developing more sensitive ultra-cold chain transport, logistics, and controls, leading to lasting innovations, says Anne Marie O’Halloran, managing director at Accenture life science’s industry X.0 and supply chain practice.
One example: the application of smart freezers. If one breaks down, a notification is issued, stating the freezer’s temperature is rising or a door has been left open. This technology isn’t necessarily new, but placing it in distribution, fulfillment, and manufacturing centers is, she adds.
Boosting Forecast Accuracy
Forecasting supply and demand takes on additional complexity when dealing with many pharmaceutical products, Chitanand says. While supply chain leaders need enough inventory on hand to meet anticipated demand, holding on to excess inventory means risking it will expire before it’s used.
This challenge will grow more pronounced as medicines become more personalized. For example, new cancer therapies have smaller patient populations because they treat a specific subcategory of the disease, says Barry Heavey, managing director in Accenture Life Sciences. The treatments are expensive to develop and manufacture, so it’s critical to ensure they’re not stalled by any one part of the supply chain.
Artificial intelligence can enhance visibility to create more real-time forecast capabilities, Hong says. This helps companies pivot in response to demand swings.
Where’s the Product?
Tracking and tracing solutions also are evolving to boost security and visibility. "Previously, a package was tracked by its shipping date and when it was out for delivery," says Scott Fata, principal director, supply chain, with Accenture. "Now, packages must be individually tracked by various security monitors."
For instance, tilt monitors can track whether a box that was supposed to sit right-side-up has been placed on its side, while temperature monitors ensure packages are at the correct temperature.
This is key, as therapies like mRNA, used in some COVID-19 vaccines, as well as with cell and gene therapies, often must be transported at temperatures ranging from -76° to -124°F (-60° to -80° C), Fata says. While companies are researching ways to reduce the need for ultra-cold chain transportation and storage, in the meantime, supply chain professionals must ensure product integrity and stability while remaining within existing technology and regulations.
Companies also are beginning to use predictive analytics to proactively reroute deliveries or change shipment approaches based on upcoming storms or shutdowns, Fata adds.
Handling Labor Shortages
Even as technology evolves, robust supply chains rely on people. While the tight labor market isn’t unique to biopharmaceutical and pharmaceutical products, the potential impact of too few employees, including delays or inaccuracies, can be significant.
"If a healthcare provider doesn’t receive the right product at the right time, it can delay care," Chitanand says.
To accelerate operations, ensure accuracy, and leverage data, more organizations are embracing automation, including mobile robots. The global market for logistics robots is expected to grow by more than 16% annually through 2028, when it will hit $17.8 billion, according to Fortune Business Insights.
In addition, tools like machine learning and natural language processing "will be the key to minimizing human error and leveraging operational data to generate strategic insights and improve productivity and accuracy of processes," according to a Deloitte report titled Intelligent Drug Supply Chain: Creating Value from AI.
Even as the pharmaceutical supply chain faces numerous challenges, it’s also advancing to address them and ensure medicines and other therapies safely and quickly reach their intended users.
The companies profiled here are leading this advance.
Locus Robotics
Solving Pharma Challenges with Robots
Locus Robotics, based in Wilmington, Massachusetts, offers its clients deep expertise in warehouse automation and logistics for many industries including healthcare and bioscience. To start, its founders come with extensive logistics and distribution experience, successfully starting and running their own 3PL that boasted KIVA robots before venturing out on their own to create Locus. Their experience shows in the sophisticated order management capabilities incorporated within Locus’s solutions.
"We understand the intricacies in the way orders are built, sent out, picked, and put away," Chitanand says.
Healthcare and life sciences logistics are major focuses of the company. "We understand the segment’s unique challenges and continue to invest in and add functionality that enables on-time healthcare fulfillment and business continuity," Chitanand says.
Locus’s user interface and reporting systems help maintain product traceability and capture serialization requirements while ensuring near-perfect accuracy on every order fulfilled. Locus works diligently to meet DSCSA (Drug Supply Chain Security Act) compliance and serialization requirements.
Locus also has developed superior navigation tools to guide its robots, ensuring they boost productivity, decrease cycle time, and operate safely. "We excel at developing the software that controls the robots," Chitanand says.
When starting to work with a client, Locus engineers typically review the company’s operational volume and primary challenges they face. Locus may initially recommend changes that aren’t related to robotics, such as "5-S’ing," or making sure a facility is organized for efficiency and effectiveness, Chitanand says.
If deploying in a greenfield facility, Locus will review the operational goals and draw from past experiences and best practices. Customers with the opportunity to design their fulfillment space around digital fulfillment technology are able to achieve the maximum throughput possible from day one. Since Locus does not require any fixed infrastructure, operators of both existing and new sites rest comfortably knowing they have the flexibility to adapt their sites as new trends emerge.
To improve the performance of the client’s operations, Locus calculates the number of robots that are needed. It’s not unusual for a small fleet of robots to double or triple productivity, Chitanand says.
A next step is estimating the return on the organization’s investment. From a capital investment perspective, most Locus solutions tend to be more attractive than large, legacy systems, Chitanand says. To start, clients can lease the robots through a Robots-as-a-Service (RaaS) model, rather than commit millions to a structured automation solution.
Once implemented, the robots work collaboratively alongside employees. Locus drastically reduces the walk time of each associate by having associates stay within zones while a fleet of robots travels between pick destinations and the packing station. This allows associates to concentrate on what they do best: picking. The approach has proven to boost productivity anywhere from two to three times for Locus customers.
Benefits of collaborative robotics go beyond productivity increases. Robots can enhance workplace safety and diminish worker fatigue by removing the need to push heavy carts and walking time. With Locus, associates on average walk 17% less than without. In addition, Locus customers have seen up to a 50% reduction in accidents and one medical distributor dropped their accident count to zero after implementing Locus.
Locus has been implementing robotics solutions across Fortune 500 medical distribution, medical device companies, and a prominent health system. In all cases, the solution integrated with the facility’s warehouse management system and significantly increased productivity.
With the increase in adoption of robotics in the healthcare space, Chitanand remains confident that robotics will solve significant logistics challenges in the healthcare/pharma space.
Controlant
Servicing the Cold Chain
The $300-billion temperature-controlled supply chain is ripe for change, Herjolfsson says. And Controlant’s mission is to do just that. From its headquarters in Iceland, Controlant provides Cold Chain as a Service® through a blend of hardware, including wireless and reusable IoT tracking devices, supply chain visibility software, and 24/7 monitor and response services.
Controlant’s solution departs from the passive technologies long used to monitor the temperature and other attributes of goods in transit. These often failed to provide the real-time visibility and data supply chain professionals require to enhance decision-making and optimize supply chains.
In contrast, Controlant takes a three-pronged approach to cold chain monitoring, Herjolfsson says. It starts with Controlant’s real-time IoT data loggers. About the size of a cell phone, these can be placed within a box or pallet—wherever the customer feels will work best—and then remains with the product throughout its journey. Supply chain professionals can monitor their products’ location, temperature, humidity, and other attributes in real time, using Controlant’s proprietary supply chain monitoring platform.
This comprehensive view enhances decision-making. For instance, a pharmaceutical supply chain leader may decide to search for an alternate transportation route if the data reveals delays or multiple excursions on the route initially chosen. (Any temperature reading outside ranges recommended in the manufacturers’ package inserts is considered a temperature excursion, according to the CDC.)
In addition, having this information available in real time means fewer products must be discarded because they fell outside temperature or other parameters. About $35 billion in biopharmaceutical products are lost annually because of failures in temperature-control logistics, according to IQVIA.
While the technology is essential, Controlant’s experienced service professionals also play a key role in monitoring clients’ shipments around the clock, and across the globe. "Our service team can act as our customers’ eyes and ears," Herjolfsson says.
Committed to Sustainability
Another distinguishing capability is Controlant’s commitment to sustainability. Not only does the visibility it provides reduce the risk of waste, but its data loggers can be reused multiple times. Historically, many data loggers have been disposed of after one use, once a supply chain journey concluded. During the 2009 H1N1 (swine flu) pandemic, Controlant’s technology was used to safeguard the distribution of critical vaccine supplies across Iceland, while also keeping pharmaceutical supply chain waste to .05%. And as Controlant monitors COVID-19 vaccines around the world, it has successfully delivered 99.997% of the needed pharmaceuticals to their intended location without any quality issues.
Several years ago, Controlant began working with a wholesale pharmaceutical distribution center that had been using a mix of business tools to track inventory and product quality conditions. This left management with disjointed visibility into its supply chain and goods, as well as little ability to prevent product damage or waste.
By working with Controlant, the company was able to proactively collect data from multiple systems and capture real-time shipment information so they could intercept at-risk shipments before the products spoiled.
And by automating communication and facilitating data sharing between departments, the Controlant solution provides a comprehensive view of the company’s operations, reducing errors and cutting supply chain waste by more than 90%.
With a history that spans several pandemics, Controlant has gained tremendous insight into the cold chain and pharmaceutical logistics. Its customers can leverage this expertise to boost their own supply chain visibility and resilience, and enhance efficiency and decision-making.
Tucker Company Worldwide
Dedicated to Quality
For more than 40 years, Tucker Company Worldwide, the oldest privately held freight brokerage in the United States, has been serving the North American healthcare sector. Along the way, it has developed the expertise and implemented the ISO 9000 processes that allow it to meet or exceed the safety standards required to transport life sciences products.
Jeff, chief executive officer and a member of the third generation in the business, started in the business as a kid. He and his brother Jim, now president and chief operating officer, would clean the company’s offices on Saturdays.
Tucker Company, which started 60 years ago, has leveraged its initial focus on moving heavy and over-dimensional loads, which often require extra security and other protective measures, and applied this expertise to the life sciences sector.
As part of this, the Tuckers committed to working only with those trucking companies that show a similar dedication to quality and safety. Of the approximately 300,000 trucking companies across the United States, Tucker has curated a list of just several dozen that it calls on when transporting life sciences and pharmaceutical projects.
"We’ll first try new carriers in easier elements," Jeff says. "Only if they’re near perfect will we test them in pharmaceuticals."
In addition to identifying truckers that can best handle these products, Tucker works with clients to evaluate the performance of different transportation lanes. To guide their evaluation, Jeff and his colleagues use good distribution practices, or GDP.
"These are generally understood as the quality standards that should govern the handling of life sciences products, and are published by the World Health Organization," he adds.
Compliance with GDP means meeting a range of requirements. These include ensuring medicines in the supply chain are stored in the right conditions at all times, including during transportation; that contamination by or of other products is avoided; and that the right products reach the right addressee within a satisfactory time period.
In addition, the distributor needs to have in place a tracing system that enables it to locate faulty products.
Jeff also helps shippers evaluate their contracts with trucking carriers. One red flag is a contract that’s signed and returned with no red lines.
"You want a healthy, vibrant conversation at the time the contract is forwarded, ensuring the parties thoroughly understand their roles," he says, noting that even thoughtful agreements—many of which extend for dozens of pages—can have provisions that might not make sense. "It you don’t discuss them, they can become problems," Jeff says.
Tucker Company prides itself on expertly serving hard-to-serve industries, Jeff says. That includes consumer electronics and temperature-controlled food and beverages, as well as the life sciences and pharmaceutical industries.
"Pharma products are really exciting and the quality of transportation is critically important," he notes. "We are proud of our incredible record transporting them."